Amy talks about access in this week’s installment of Drug Development Wednesday. What is access, and why is it that nearly all American cystic fibrosis patients have access to CFTR Modulators, while access is limited internationally.
When I was a junior in college I lived, studied and worked in Europe, and it was one of the most amazing experiences of my life. Talk about pushing myself – even though it was still western culture, there were so many nuances of living outside of my home country that I had to learn and navigate on a daily basis. And that’s without CF.
When my vest broke while living in Europe, I couldn’t get it fixed there – I had to send it back to the States for repair. As a result, I had the opportunity to learn about how different the healthcare system was in Europe compared to the US – and why the vest wasn’t available to any CF patient in Europe unless they paid cash. Clearly, differences existed – and still exist – between health insurance models across the world.
Fast forward 17 years and I’m still interested in the differences between the health insurance landscape across the world. Particularly, I really want to understand why 99% of all American who fit the FDA indication for Vertex CFTR modulators have access to the drugs – most for low or no cost out of pocket.
But in UK, CF patients still don’t have access to Orkambi 3.5 years after American FDA approval (aside from severe cases in the UK – in 2017 243 patients received the drug). Why this difference?
Today’s article is the first in a two-part series discussing to CFTR modulators. First, we will discuss the US and next article we will discuss Europe.
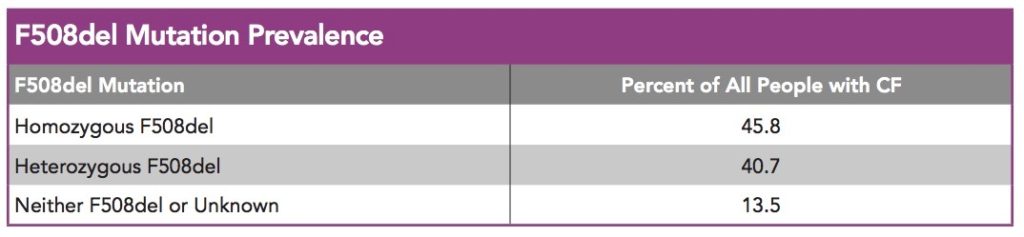
Before I go further, this isn’t a “ra-ra” piece about how amazing the US healthcare system is. The US insurance system of course has flaws. I’m simply focusing on this drug development topic because it’s such an immediate, hugely impactful reality for the ~15,000 or so double DF508 CF people who have access to CFTR modulators in the US as well as the 4,856 double DF508 CFer’s in the UK who do not.
So why do patients in the United States have such early and wide access to CFTR modulators compared to the UK and other countries around the globe? I think it comes down to three things:
(1) American culture and what the country as a whole chooses to prioritize.
(2) The US has a public/private hybrid of health insurance in the US provides competition that benefits patients
(3) Pharmacoeconomics
Priorities
It’s no secret that the United States pays more for healthcare per capita than any other developed nation. There is a long list of reasons as to why this is, but included in this list without question is the prioritization for choice and innovation. If you want to be able to choose what physicians to see, what medications to take – and to have the absolute latest and greatest that science has to offer, you’re going to pay for it. I’m definitely not saying that every person in the US gets choice and access to the latest and greatest medical treatment. But, on the whole, most do.
Also, many Americans have a general distrust of a central authority deciding whether or not they can have something based on cost– whether it’s a medical procedure or a medication. The Food and Drug Administration is tasked with approving a drug for use in the US based on efficacy and safety alone – not cost, not value. Let’s see how this applies to the American CF population.
43.5% of people with CF in the United States have Medicaid for their health insurance – the joint federal- state health insurance for those who are under a certain income level. Medicaid is free to patients, for the most part. “Medicaid requires states … to cover all drugs that the FDA approves (with limited exceptions)” for little or no cost to the patient. There are some rare limitations here and there, but on the whole, this is the state of Medicaid in the US. So, for the past 3.5 years that Orkambi has been on the market and the 9 months Symdeko has been on the market, the US has given the poorest among us to have access to choice and innovation when it comes to prescription medications.
Public / Private Hybrid of Health Insurance
59.5% of US CF people are on private health insurance (PPO, HMO, EPO, etc). Patients with private health insurance are able to use co-pay cards to significantly reduce the cost medications (include drugs from Vertex) – allowing for not only broad access to CFTR modulators but at a very low price around $30/month. There are states attempting to limit co-pay card adjudication at the pharmacy level for private insurance companies (there are ways around this, like sending in a rebate directly to the drug manufacturer, or stating that co-pay card use cannot count towards deductibles and out of pocket max limits for insurance), but for the most part, orphan drugs and specialty drugs are excluded. If this changes, of course, this will be a problem for access to CFTR modulators in the future.
9.9% of all CFer’s are on Medicare (federally funded insurance plan for those who are age 65 and older or for those who are permanently disabled). The CFF registry doesn’t break down if CFer’s on Medicare also have Medicaid, or patients with Medicare and Low Income Subsidy – both programs dramatically reduce drug costs to little or no co-pay. I have submitted an inquiry to the CFF registry and once I hear about, I will get back to you with the data. Sidebar: the CF Registry should be open source so our community can access and learn from the data.
The CFF registry states that 0.7% of patients in 2016 went without health insurance. Thankfully, there are an abundance of programs for uninsured to receive medications. Not a perfect system – everyone should be able to have health insurance. But thankfully this # is quite low in our CF population.
For the few who have Medicare for their insurance and don’t qualify for Medicaid and low income subsidy, cost of CFTR modulators can be high because the federal government doesn’t allow patients with government-funded health insurance to use manufacturer co-pay cards to reduce out of pocket drug costs. There are resources such as foundations (Healthwell is one of several) that help to offset costs for Medicare folks without Medicaid and LIS. Sometimes, there is an income limit to qualify for the foundation and foundation assistance to cover drug co-pays can also be capped if the particular foundation chooses.
It’s important to emphasize that currently with double DF508 modulators on the market, statistics show around 1500 people in the US with CF have Medicare (10% of 15,000 double DF508 patients). It will be interesting to see how Vertex handles this population moving forward, when the 40% of American DF508 heterozygotes have access to the triple combo. My guess is that Vertex is smart enough to fund foundations for the additional 12,000 DF508 heterozygotes who will use a CFTR modulator and also have Medicare (although likely, many of these Medicare patients will qualify for Medicaid or Low Income Subsidy, dramatically reducing cost).
With 50 states in the union, CF patients are widely distributed across the country, so CF people with Medicaid as their insurance don’t overwhelm one particular state with CFTR costs.
Then we take in to account the 858 different private health insurance companies there are in the United States, and we see CF patients are spread out among various plans – therefore a single entity isn’t responsible for drug costs for the entire American population.
Remember, the CF population represents only 0.01% of the total US population. A recent survey of private health insurance plans (called payers) found that “payers seem ‘more focused on larger categories where there is more need to manage costs,” including cancer, diabetes, and hepatitis drugs. In fact, of insurance companies surveyed, orphan drugs are only about 4-6% of spending.
So why not cover CFTR modulators? No brainer there.
Pharmacoeconomics
This is my favorite part that I rarely hear discussed – pharmacoeconomics. That term can mean many things, but in the drug industry, it refers to how medications save money in other areas of the healthcare system. The economic scope of it all.
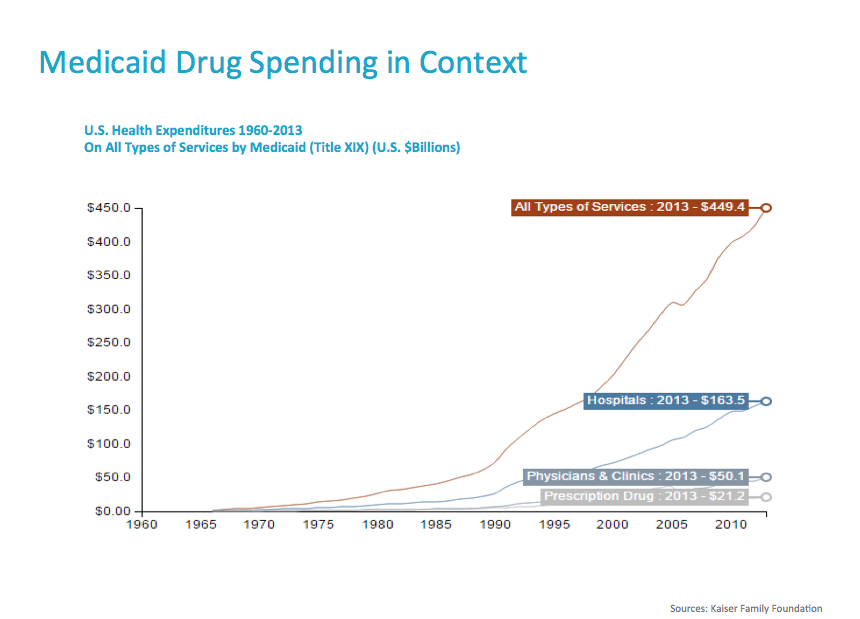
A few fun facts before we continue: did you know that prescription drugs account for less than 20% of all healthcare spending in the United States? That’s right. Hospital care and physicians represent 52%. In fiscal year 2014, prescription outpatient drug costs were only 5% of total Medicaid spending.
According to Optum, “members [patients who have a particular health insurance] with rare diseases tend already to have high claims costs. These can include frequent episodes of acute care — office visits, ER, hospital admissions, home care, etc. This means that even a very costly new therapy could be a ‘net lower cost’ alternative, especially if the therapy helps to reduce acute care costs. Depending on the situation, the plan may even find it worthwhile to be very proactive in persuading network physicians to prescribe the new medication.”
The longer CFTR modulators are on the market and more widely they are used, the more our community learns how modulators save costs of CF care in other areas. Orkambi has been shown to decrease exacerbation frequency in CF, including rates of hospitalization. That’s a cost saver!
As you can see, there are several factors that are in play that allow 99% of eligible American CF patients to have access to CFTR modulators – many for little or no cost. But all CF patients across the globe should have access and it’s unacceptable that our brothers and sisters in the UK currently don’t have access to Orkambi and Symdeko.
Stay tuned for part 2 discussing why every day, 4,865 CF patients go without life-saving medications in the UK!
**An edit was applied to allow the images to appear clearer than the original post. A technology error had led images to appear blurry on some devices.
The views expressed on any guest column [Drug Development Wednesday], are that of guest contributors, and not necessarily those of Gunnar Esiason or the Boomer Esiason Foundation. Nothing in guest columns should be considered medical advice; such advice can only be given by a physician who is experienced with cystic fibrosis. The Boomer Esiason Foundation, Gunnar Esiason, and guests cannot be held responsible for any damage which may result from using the information on this website without the permission of your medical doctor.