The 2010’s saw the dawn of the modulators. In a remarkable feat of drug development, four cystic fibrosis-specific medications came to market from the test tube: Kalydeco, Orkambi, Symdeko and Trikafta.
(If you want to blow your mind, look at the generic names of the drugs included in the modulators – Ivacaftor, Lumacaftor, Tezacaftor, Elexacaftor – you’ll notice they all include CFTR).
I cannot emphasize enough that four drugs making it through trials, the FDA, and US patients being allowed wide access to them is one of the most amazing feats of the biopharmaceutical industry in recent memory.
But where are we headed next? The 2010’s didn’t really see too much more as far as cystic fibrosis-specific drug development is concerned. Sure, reformulations of existing medications like Cayston, an inhaled antibiotic, and Relizorb, a feeding tube specific enzyme cartridge, hit the market, but beyond that we’ve been on the backs of the modulators, and as we well know, they don’t work for everyone with cystic fibrosis.
“Next” is going to be a massive undertaking. Any new disease-modifying drug that comes to market will have to, at the very least, tie the Vertex drugs as far as efficacy goes – that benchmark has been set quite high at 13.8% mean increase in FEV1 – to trigger a communitywide standard of care switch or target the remaining portion of the population who aren’t able to use our modulator arsenal.
RNA
RNA treatments will likely be the “breakthrough” that we’re going to be talking about next. RNA medications that target CFTR are mutation agnostic, meaning they will be suitable for anyone with cystic fibrosis regardless of mutation. The way they work is a little bit like science fiction. It looks like they will likely be inhaled treatments (at least the candidate from Translate Bio is inhaled).
In the interest of being simple, patients will inhale the mRNA that will carry messages to the part of the body that creates CFTR protein. You, me, and everyone else with CF creates dysfunctional CFTR protein, so when the medication is introduced into the body, instead of creating dysfunctional CFTR protein, the inhaled mRNA will tell our bodies to create functional CFTR.
This is different from modulators in that the Vertex meds fix the dysfunctional protein after the body has created it. RNA medications will be mutation agnostic because they aim to fix CFTR before it’s created.
Congratulations, you now have an undergraduate degree in cellular biology!
RNA therapy is going to be big business for whoever is the first to get it into CF because of the way drug patenting works. Remember, big business is critical to the success of our drug development pipeline because of the way Venture Philanthropy is structured. Whenever one of the medications that Cystic Fibrosis Foundation has invested in hits its target, the ensuing royalty revenue or sale supercharges our pipeline for future drug discovery. The next generation of cystic fibrosis medications will be thanks to the success of the modulators over the past decade. The hope is that this strategy can continue, with each ensuing generation of medications due largely to the success of past investments into biopharma.
So yes, while we had had a massive Venture Philanthropy success in the early 2010’s, I expect one coming from somewhere other than modulators in the 2020’s. Maybe RNA will be the place? CFF has made considerable investment into mRNA technology.
RNA therapy is exciting because it could be the disease modifier that the rest of the population is waiting for. Will all of us switch from modulators to RNA therapy, will it be complementary to modulators, or will it even work? That all remains to be seen. Translate Bio has had some decent success in early stage trials, and most importantly, everyone remained safe and intact. Remember, the critical marker for success is if RNA therapy’s efficacy is greater than or equal to what we currently use or if these medications prove to be good enough for people who do not have other options. If you’d like to look into the companies pursuing RNA treatments, check out Translate Bio and Arcturus Therapeutics.
The Antibiotic Issue
Over the next several years you’re going to read more and more about the antibiotic problem, and my expectation is that it is not going to get better any time soon. I hope I am wrong.
Resistance is on the rise and new options are not getting through the pipeline. When the odd medication does get through, the past few years have shown us that the market has not been able to support smaller entries. Big pharma has all but abandoned the antibiotic market, leaving it up to boutique firms to handle the burden.
Whether or not you’re the kind of person who has tremendous faith in the biopharmaceutical industry or absolutely loathes it, this is a critical issue for all of us. Just last week another antibiotic developer filed for bankruptcy. The problem is multifaceted.
Contrary to the way the free market (and the indirect healthcare market) works, policy makers, patient advocates, and health experts are encouraging antibiotic stewardship. Antibiotic resistance is on the rise because of the misuse of antimicrobials in humans and livestock. Stewardship encourages antibiotics as a last resort or only when necessary – we fall within the necessary category in CF.
While stewardship is proving to be one of the most effective ways to battle antibiotic resistance, the reimbursement system, however, is not set up to reward antibiotic stewardship. We are telling folks to user fewer and fewer antibiotics to preserve efficacy, but firms producing them can’t sustain a healthy business model, and thus go belly up – and that includes two separate drug makers this year alone.
As we enter the new decade, you’re going to hear a lot about the antibiotic crisis, and it’s critical that we all wave this flag. Antibiotic resistance has the power to undermine whatever breakthrough comes our way, and right now we don’t have a way to sustain progress despite a handful of exciting technologies in the works.
Keep an eye out here on the blog and over on my Twitter for some of the stuff I’ve been working on to combat this issue!
Gene-Editing
By the end of the next decade (when I’m in my late thirties – lol yikes), I think we will see the first person with cystic fibrosis dosed in a gene-editing trial. Gene therapy has been a bit of a taboo strategy in CF ever since the failures in the early 90’s, but the limited success it has had in other rare diseases is bringing its potential front and center to our condition. In fact, gene-editing has already been shown to have some success in-vitro in CF. Beyond that, Cystic Fibrosis Foundation is investing heavily in gene-editing, and speculation abounds that the $500 million the Foundation is investing is going to be primarily focused on gene-editing. Vertex has their hat in the ring as well with their partnership with CRISPR Therapeutics, and given their huge success in Sickle Cell, there is a lot of reason to be optimistic about that tech coming into CF.
Policy Adapting to Precision and Personalized Medicine + Normalizing Teleheatlh
One of the things the lackluster American healthcare system does very well is prioritize the development of orphan drugs. It’s undeniable as we we’ve seen more orphan drugs make it to market over the past few years than ever before.
That includes four cystic fibrosis-specific drugs in the past decade.
The same trend is true for personalized medications as well.
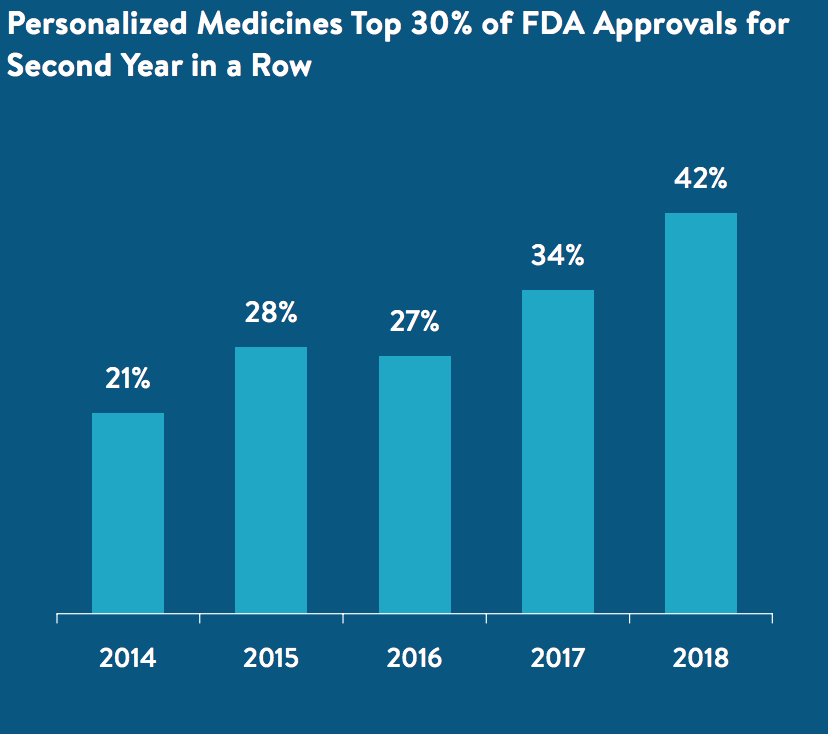
Innovation is something we can never sacrifice. Despite the progress we’ve made, we are still going to be dependent on future iterations of personalized medications. Complacency is not a luxury we can afford – especially for people still living with critical illness or people who count themselves as part of the remaining 10%.
Generally speaking, access to orphan drugs is quite good, too. Out of pockets are low as long as co-pay assistance programs are allowed to do just that… assist. It’s my hope that the drug pricing debate in the US will talk about out of pocket costs before anything else. Quite frankly, patient costs are really all that should matter initially, and right now that isn’t the case.
Our healthcare system is going to have to accept the change to precision medications from cocktail approaches sooner rather than later. We’re sort of in the midst of it now, and I know wide-reaching reforms are fun to talk about, but they aren’t super realistic in the near term. Since this is a “realistic” expectations blog, I think we will see economic incentive continue for precision medications, and I also think that reimbursement policy will eventually catch up, too. What I fear, however, is that Medicare patients in the United States will continue to be crunched by federal anti-kickback laws. We’ve discussed those policies on the blog before (click the above link on the word, “assist”), so I won’t dive into them here, but in my opinion that’s the first piece of the healthcare debate that needs to be altered to help out people living with rare disease.
I also think as telehealth is normalized, we’ll be able to bring down inpatient and hospital-based complications. Hospital error is one of the leading causes of death and injury in the United States, and hospital-acquired infections are vastly underreported for a number of reasons, so I do think that telehealth will be the kind of thing that can help bridge this gap, and bring hospital spending down. Look for legislation that will improve reimbursement and access to telehealth from the comfort of your own home in the next decade.
Alternative Transplant Methods
Transplant medicine is exploding. The prevailing strategy seems to be fighting against the potential for rejection before organs are transplanted, rather than after, and that sounds good to me – especially since we’re dealing with lungs. Lungs are the only transplanted organs that are consistently exposed to outside contaminants (air), and the immune system is kind of important. Decimating the immune system brings along a number of complications (outside the scope of what we’re talking about here), so if we can transplant lungs AND keep the immune system in tact, could we expect better outcomes for transplanted CF patients? Possibly! The way researchers are attempting to do this is by growing organs in the lab using patient stem cells. Essentially they hope to grow your own organs outside of the body and then transplant them into you when the time comes. Does it sound like science fiction? Well… it is. We aren’t exactly close to this happening quite yet, but maybe by the end of the 2020’s we will see it happen. I think it’s a long shot, though. Another potential option could be using pigs to harvest organs for transplant. It would cut down the wait time on transplant lists and hopefully result in better outcomes.
A Cure?
I’ve been saying this for a long time, but cure is a huge word in CF. Remember… CF is a condition that causes many diseases in the body to manifest – respiratory disease, pancreatic disease, liver disease, infertility, etc. etc. In the classic sense of the word, a cure would mean all of that would go away. For people with advanced CF or advancing CF, a cure would also require regenerative medicine, which at this point is very much science fiction. I think some of the above technologies will yield livable cures meaning CF would be noting more than a condition that would require periodic management. In fact, kids who are starting on modulators at a young age are already living that lifestyle! I do think, however, that we will see the first generation of kids born with CF in the next decade who will live without much of the CF you and I know today. Is that a cure? Maybe!
I hope everyone has a HAPPY HAPPY HAPPY New Year!