Happy Wednesday, folks. Today we’re introducing our first ever guest column on the blog! I asked a friend of mine with CF if she’d dive into the world of drug development using her 14 years of experience of working in the healthcare industry. Amy’s column, Drug Development Wednesday, will run every other week, and include 8-10 total posts. We’ll be looking at the lifespan of a medication – where it comes from and how it gets into a patient’s hands. I’m really excited for what Amy has to offer, especially since this will be coming from the mind of an adult living with cystic fibrosis!
I would argue that aside from the CF gene discovery in 1989, 2018 is the most exciting time to be alive with cystic fibrosis. CF patients are living longer than they ever have in human history!
But if we look at those years – 1989 to 2018 – it’s hard not to wonder what in the world has taken so long to develop drugs that treat the underlying cause of CF. 2012 saw the advent of Kalydeco to treat about 4% of CF patients – but that’s still a 23-year gap.
I was 8 years old when the CF gene was discovered and I remember vividly how excited our little community was when The Hospital for Sick Children (SickKids) in Toronto, Canada, and Dr. Lap-Chee Tsui finally found the “needle in the haystack” – the gene that caused CF.
In 1989 we were told it was only a matter of years (not decades) until the disease was cured. CURED. So…. what happened?
Like most things in life, the answer is complicated – but it’s so important for us to understand. The explanation boils down to 3 major reasons: (1) The time it takes to discover a new drug (2) The cost of developing a new drug and (3) the landscape in which the industry operates.
Time
First thing’s first – it takes 12 years for a new drug to reach the market according to a whole slew of sources including the BBC, Journal of the American College of Cardiology, and Scientific American to name a few. In fact, The Tufts Center for Drug Development states that bringing an orphan disease drug to market can take on average 2.3 years longer than a non-orphan drug, bringing us to an average of 15.1 years.
And again, for a large percentage of the CF population, including yours truly, there still is no modifier in 2018 for their mutations.
Again… what gives?
Cost
Well, we need to examine the cost of drug development. Depending upon who you talk to, bringing a single drug to market can cost anywhere between $1.2 billion to $2.4 billion. And this cost doesn’t vary if you’re developing a drug for 70 patients, 70,000 patients, or 70 million patients – the cost is nearly the same. Drug companies still have to pay for scientists, research equipment, real estate to house the scientists and equipment, regulatory liaisons to discuss clinical trial structure with the FDA, statisticians to analyze results, doctors, hospitals and patients to participate in 3 phases of clinical trials, the list goes on and on.
The pharmaceutical/biotech industry is unquestionably “tougher and riskier at nearly every stage than any other business…. The failure rate even after a [drug] candidate clears all the myriad of hurdles to reach human testing is 30 to 1.”[1] In fact, most pharma researchers work on developing medications that never get FDA approved – let that sink in. This is extremely difficult work.
So let’s pretend for a moment, you are the CEO of a pharmaceutical or biotech company and you have $2 billion dollars burning a hole in your pocket – would you take a gamble to develop a drug that would treat 70 million patients or 70,000 patients (all things equal)?
Spoiler alert: you would choose to develop the drug that treats 70 million patients. Every. Single. Time. Until recently.
Cystic Fibrosis in 1990 affected around 18,000 people in the United States (30,000 in the US , 70,000 worldwide in 2018). From a business perspective, the numbers are quite clear – who would want to invest billions developing a drug for such a small population of patients when they could develop drugs for heart disease (#1 killer worldwide) with tens of millions of patients worldwide? Or diabetes? Or asthma? No drug company is in the business of losing money – and trying to bring a drug for CF to the market would be an obvious money loser. According to the FDA, “research hospitals and universities, also lacked the capital and business expertise to develop treatments for small patient groups.” Many factors are not on the side of developing new, innovative medications to treat cystic fibrosis.
Landscape
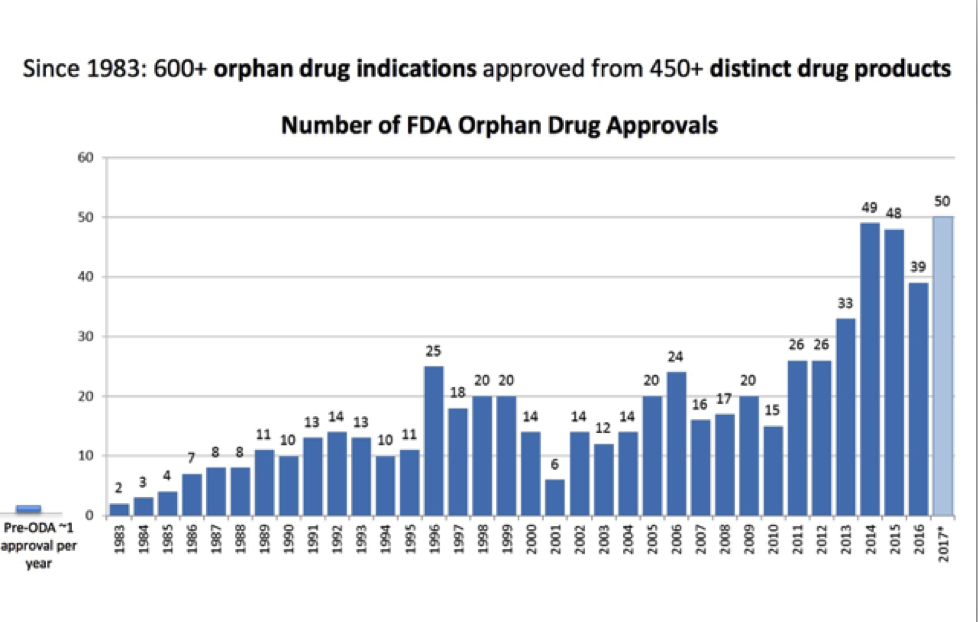
In 1983, United States Congress enacted the Orphan Drug Act to provide incentives to drug manufacturers including federal grants, tax credits and longer patent protection (amount of time a drug manufacturer has exclusive rights to market a drug) and other perks. This legislation was a game-changer! From 1983-2009, 347 orphan drugs were FDA approved – a massive improvement from 1967-1983 when 34 medications were approved (that would have fit the criteria under the Orphan Drug Act had it been in effect during those years). The legislation was a clear win for patients with orphan diseases and led to exponential growth in drugs available for those who were previously neglected.
The drug industry also hit an inflection point in the early 2000s, where essentially all low hanging fruit of mass market drugs had been picked. These multi-billion dollar “blockbuster” medications were going off patent at this time (which meant cheaper generics were available, plummeting revenues for drug companies who discovered the original drugs). Pharma/biotech companies had to search for the next big thing, and some companies were smart enough to see potential in smaller, more niche diseases; and even orphan diseases – including cystic fibrosis.
But there are over 6,000 orphan diseases in the world. How does CF grab the attention of researchers ahead of other illnesses?
In 1998, Robert Beall, then CEO of the Cystic Fibrosis Foundation, pioneered a venture philanthropy model to further incentivize drug development for CF. The CFF would take dollars raised by American CF patients, their family and friends, and use the money to incentivize private corporations to conduct research in CF. Essentially, cut the risk for pharma/biotech companies of investing millions of dollars of their own money.
Not many drug companies, however, were interested in testing the venture philanthropy waters. But Aurora Biosciences decided to take a chance on cystic fibrosis – and in 2000, the CFF agreed to give $47 million to Aurora in “the largest contract ever awarded a for-profit business by a non-profit health organization.”[2] History was made; and work on Kalydeco accelerated.
In 2001, Vertex acquired Aurora Biosciences Corporation for $592 million. 11 years later, Vertex’s Kalydeco was FDA approved in the United States, with Orkambi approved in 2015 and Symdeko in 2018.
There is no question that the CF community wants a cure yesterday. However, in understanding the massive headwinds our tiny disease has faced in drug development the past few decades, it’s nothing short of a miracle that immense progress has been made in treating the underlying cause of the disease. We still have a long way to go in CF drug development, Kalydeco, Orkambi and Symdeko make 2018 by far the best time to be alive with CF – and our community’s story is without question the best story in medicine today. Fight On!
[1] Werth, Barry. The Antidote. New York: Simon & Schuster, 2014. Print.
[2] Werth, Barry. The Antidote. New York: Simon & Schuster, 2014. Print.
The views expressed on any guest column [Drug Development Wednesdays], are that of guest contributors, and not necessarily those of Gunnar Esiason or the Boomer Esiason Foundation. Nothing in guest columns should be considered medical advice; such advice can only be given by a physician who is experienced with cystic fibrosis. The Boomer Esiason Foundation, Gunnar Esiason, and guests cannot be held responsible for any damage which may result from using the information on this website without the permission of your medical doctor.